價格:免費
更新日期:2018-10-13
檔案大小:8.3 MB
目前版本:2.6
版本需求:需要 iOS 11.4 或以上版本。與 iPhone、iPad 及 iPod touch 相容。
支援語言:英語
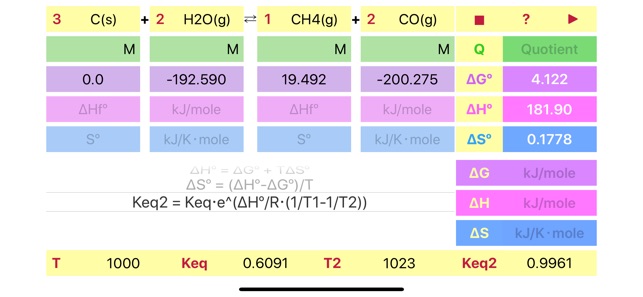
Reaction Thermodynamics helps in evaluation of main thermodynamic parameters of a chemical reaction. Application calculates the following parameters:
Standard free Gibbs energy, enthalpy and entropy from formation energy parameters.
Reaction quotient (Q) from concentrations and reaction stoichiometry.
Non standard energy parameters (dG, dH, dS) from standard ones, quotient and temperature.
Reaction equilibrium constant, Keq.
Van't Hoff relation to follow change in equilibrium constant with temperature assuming constant reaction enthalpy.
Pressing yellow buttons (dG°, dH°, S°) will initiate calculation of formation parameters based on reaction stoichiometry. The final results will be standard reaction energies. Alternatively, if formation parameters are unknown, the standard parameters can be filled in directly.
Scrolling to the required formula and pressing run button initiates calculation according to the formula and set results in the appropriate fields (Keq, dG, dH, dS, Keq2) and in the fields for standard parameters. Please pay attention to the parameters needed for solution of the formulas. The empty fields will blink to attract special attention. Additionally, the fields where the formula results are set will blink too.
Temperature (in Kelvin only) can be set in the field next to reaction formula.

The default units are kJ/mole for dG and dH, kJ/Kmole for dS. Keq units depend on reaction stoichiometry and may be unitless or any mixture of Molar and atm.
Important!!! If compound doesn't participate in equilibrium calculation (like in case of water in water solution or solid in gas phase reaction) please set concentration of the compound to 1 or set its stoichiometric coefficient to 0 (depends on case) to ensure proper Quotient calculations. Ask developer directly in case of a ambiguity.
Reaction stoichiometric parameters and concentration/pressure units can be changed by pressing the corresponding fields.
Calculating standard Gibbs free energy changes
For the general reaction aA + bB = cC + dD
ΔG°rxn = cΔGf°(C) + dΔGf°(D) - aΔGf°(A) - bΔGf°(B)
Example: Calculate the Gibbs free energy for the following reaction at 25 °C.
Cu (s) + H2O (g) -> CuO (s) + H2 (g)
ΔG°rxn = ΔGf°(CuO (s)) – ΔGf°(H2O (g)) = (–129.7 kJ/mol) – (–228.6 kJ/mol)
= 98.9 kJ/mol
ΔGf° = 0; for elements in their standard state by definition.
At equilibrium, ΔG = 0!

支援平台:iPhone, iPad
